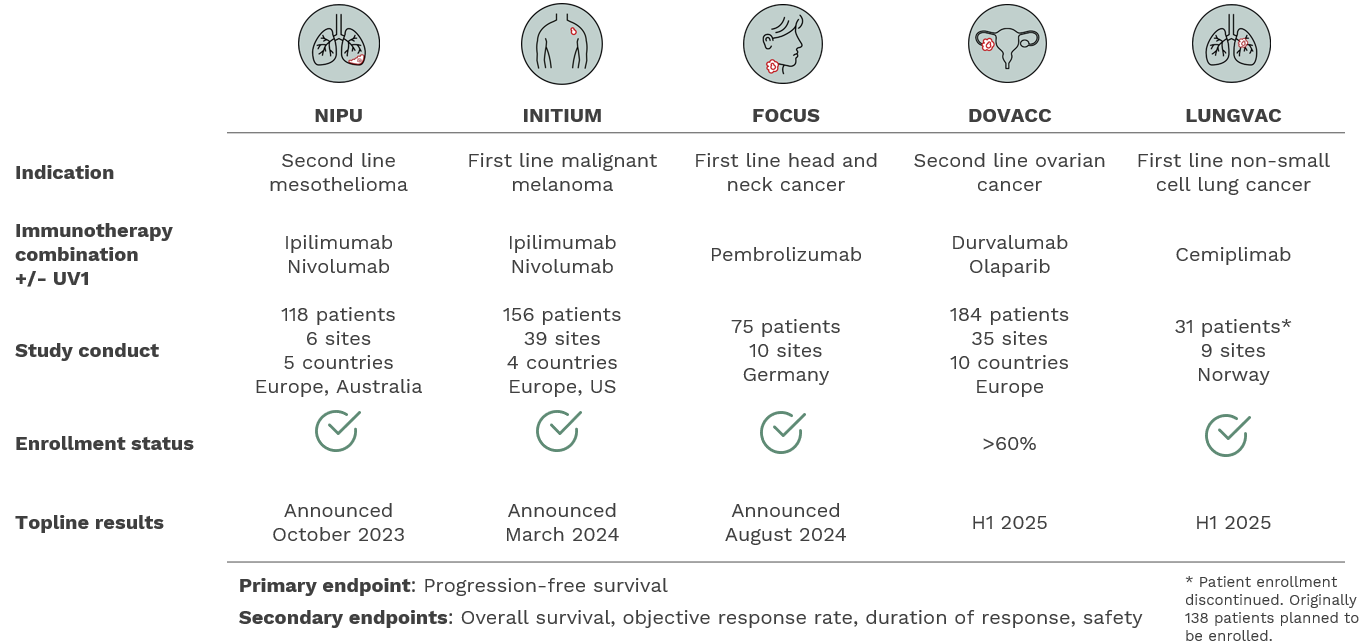
Ultimovacs has an extensive development program with five Phase II studies in different indications and immunotherapy combinations, enrolling more than 560 patients.
INITIUM – malignant melanoma
INITIUM is an Ultimovacs-sponsored randomized, open-label, multi-center Phase II trial in which the off-the-shelf cancer vaccine UV1 was evaluated in combination with the checkpoint inhibitors ipilimumab and nivolumab for first-line treatment of patients with unresectable or metastatic malignant melanoma. 156 patients were randomized between June 2020 and July 2022. The study was conducted at 40 sites in 39 hospitals across the U.S., UK, Belgium, and Norway.
Ipilimumab and nivolumab are considered the most efficient treatments for these patients. The trial was designed after the registrational trial for ipilimumab and nivolumab, CHECKMATE-067, which reported a median PFS of 11.5 months. The primary endpoint in the INITIUM trial was progression-free survival. Secondary endpoints include overall survival (OS), objective response rate (ORR), duration of response (DOR), and safety.
In September 2022, Ultimovacs initiated a supplementary single-arm study to the INITIUM trial. The study was fully enrolled in October 2023 with a total of 21 patients. The single-arm study was designed to describe the mechanisms leading to improved clinical effects in patients treated with UV1 vaccination. The single-arm study will provide in-depth data on biological activity and mode of action of the T cells induced by the UV1. All patients will receive experimental treatment (i.e., the triple combination of UV1, ipilimumab, and nivolumab). Data collected from the supplementary study will not be part of the primary and secondary endpoint analyses of INITIUM. Six patients in the INITIUM study will also be part of the INITIUM supplementary study, for a total of 27 patients.
The topline results from the INITIUM trial reported in March 2024
Ultimovacs announced in March 2024 that with the 18-month minimum follow-up of all patients, the trial did not meet the primary endpoint of improved progression-free survival (PFS). The median PFS was not reached in either arm, and the Hazard Ratio (HR) between the arms for PFS was 0.95. Evaluation on secondary endpoints did not show a difference in overall survival, objective response rate or duration of response. UV1 maintained its positive safety profile with similar safety and tolerability as observed in the control arm.
NIPU – mesothelioma
NIPU is a randomized, multi-center Phase II trial that assesses the universal cancer vaccine, UV1, in combination with the checkpoint inhibitors ipilimumab and nivolumab as second-line treatment in metastatic malignant mesothelioma. The study was designed to assess the impact of UV1 on progression-free survival (PFS) in patients with metastatic pleural mesothelioma after progression on first-line standard platinum doublet chemotherapy. Prof. MD PhD Åslaug Helland is the principal investigator for the trial, which was sponsored by Oslo University Hospital (OUS). Bristol-Myers Squibb and Ultimovacs each entered into agreements with OUS to support the preparations and execution of the trial.
The first patient in the NIPU trial was treated at the Oslo University Hospital in June 2020, and the last patient was enrolled in January 2023. The study was conducted in 118 patients in five countries (Norway, Sweden, Denmark, Spain, and Australia). Half of the patients in the trial were treated with the combination of UV1, ipilimumab and nivolumab and the other half with ipilimumab and nivolumab.
The topline results from the NIPU trial was reported in June 2023
The NIPU results demonstrated that patients receiving UV1 vaccination as add-on to nivolumab and ipilimumab experienced an increased objective response rate and a clinically meaningful prolonged survival. The data provides a foundation for further advancing clinical development with UV1 vaccination in mesothelioma patients. The results from the NIPU trial were shared in a late-breaking abstract and as an oral presentation by the Principal Investigator at the ESMO Congress 2023 in Madrid in October.
Based on blinded independent central review (BICR), the study did not meet the primary endpoint of PFS. Investigator assessment, a pre-defined supportive analysis of the primary endpoint performed by specialized radiologists at the study hospitals, showed a statistically significant positive PFS benefit for the patients in the UV1 arm.
Updated OS results were reported in September 2024.
FOCUS – head and neck cancer
The FOCUS (First-line metastatic Or recurrent HNSCC/Checkpoint inhibitor UV1 Study) trial is an investigator-sponsored, randomized Phase II clinical trial that enrolled patients with recurrent or metastatic PD-L1 positive head and neck squamous cell carcinoma. The trial was conducted at 10 sites across Germany and led by principal investigator Prof. Mascha Binder, M.D., Medical Director and Head of the Immunological Tumor Group at University Medicine Halle, Germany, who is a renowned oncology clinician and researcher specializing in the analysis of immuno-oncology treatments and their interaction with tumor tissues.
The trial evaluated the addition of UV1 to a standard of care treatment with PD-1 checkpoint inhibitor pembrolizumab as compared to pembrolizumab monotherapy. A total of 75 patients indicated for treatment with pembrolizumab was enrolled in the FOCUS study, randomized 2-to-1 so that 50 patients received UV1 and pembrolizumab and 25 patients received pembrolizumab alone. The primary endpoint of the study was the progression-free survival rate at 6 months. The first patient in the FOCUS trial was treated in August 2021, and enrollment was completed in August 2023.
The topline results from the FOCUS trial reported in August 2024
The Phase II trial did not meet its primary endpoint of improved progression-free survival (PFS). In addition, the data did not show clinical benefits on overall survival. The safety profile was consistent between the two arms and in-line with previous UV1 studies, confirming the good safety and tolerability profile for UV1.
DOVACC – ovarian cancer
The DOVACC (Durvalumab Olaparib VACCine) trial is designed to evaluate Ultimovacs’ proprietary UV1 cancer vaccine in combination with AstraZeneca’s durvalumab, a PD-L1 checkpoint inhibitor and its PARP inhibitor, olaparib, the maintenance therapy for BRCA-mutated, advanced ovarian cancer. The trial is conducted at 35 hospitals in 10 European countries. The first patient was enrolled in December 2021. Topline data on the primary endpoint is expected in H1 2025.
The second-line maintenance study will enroll patients with high-grade BRCA-negative ovarian cancer after partial or complete response following the second round of chemotherapy. The study includes three arms treating a total of 184 patients. The first arm will enroll 46 patients receiving the PARP inhibitor olaparib. The 46 patients enrolled in the second arm will receive olaparib and the checkpoint inhibitor durvalumab. The third arm will include 92 patients that will receive Ultimovacs’ UV1 vaccine in combination with both AstraZeneca drugs. The primary endpoint is progression-free survival (PFS) in the treatment arm with PARP inhibitor olaparib monotherapy, versus PFS in the triple combination treatment arm. Under the terms of the collaboration, Ultimovacs will provide its UV1 vaccine and AstraZeneca will provide the PD-L1 and PARP inhibitors for the study.
DOVACC is a multi-center, multinational, randomized Phase II clinical collaboration trial with the Nordic Society of Gynaecological Oncology – Clinical Trial Unit (NSGO-CTU), the European Network of Gynaecological Oncological Trial Groups (ENGOT) and AstraZeneca. The trial is sponsored by the NSGO, the leading gynaecological oncology research society in the Nordic and Baltic regions.
LUNGVAC – non-small cell lung cancer (NSCLC)
The LUNGVAC trial is a multi-center, randomized, open-label, investigator-sponsored trial assessing the safety and efficacy of UV1 in combination with cemiplimab versus cemiplimab alone in NSCLC patients with advanced or metastatic disease. The trial treats patients with tumors classified within the adenocarcinoma or squamous subgroups of NSCLC, where at least half of the tumor cells express the PD-L1 antigen and who have not previously received pembrolizumab treatment. These subgroups represent approximately 1/3 of all advanced and metastatic NSCLC patients. Professor Odd Terje Brustugun is the principal investigator for the trial, which is sponsored by Drammen Hospital, a leading oncology research center in Norway. The trial was originally to enroll approximately 138 patients at 9 clinical centers in Norway.
In September 2024, Ultimovacs announced that the company has agreed with the investigators conducting the LUNGVAC trial to discontinue patient recruitment. The decision was driven by very slow recruitment in the study, which is primarily due to new treatment options available to NSCLC patients. All 31 patients that have been enrolled in the LUNGVAC study since 2022 will be treated and followed up as per the trial protocol. The first patient was enrolled in October 2022, and topline readout is expected in H1 2025.