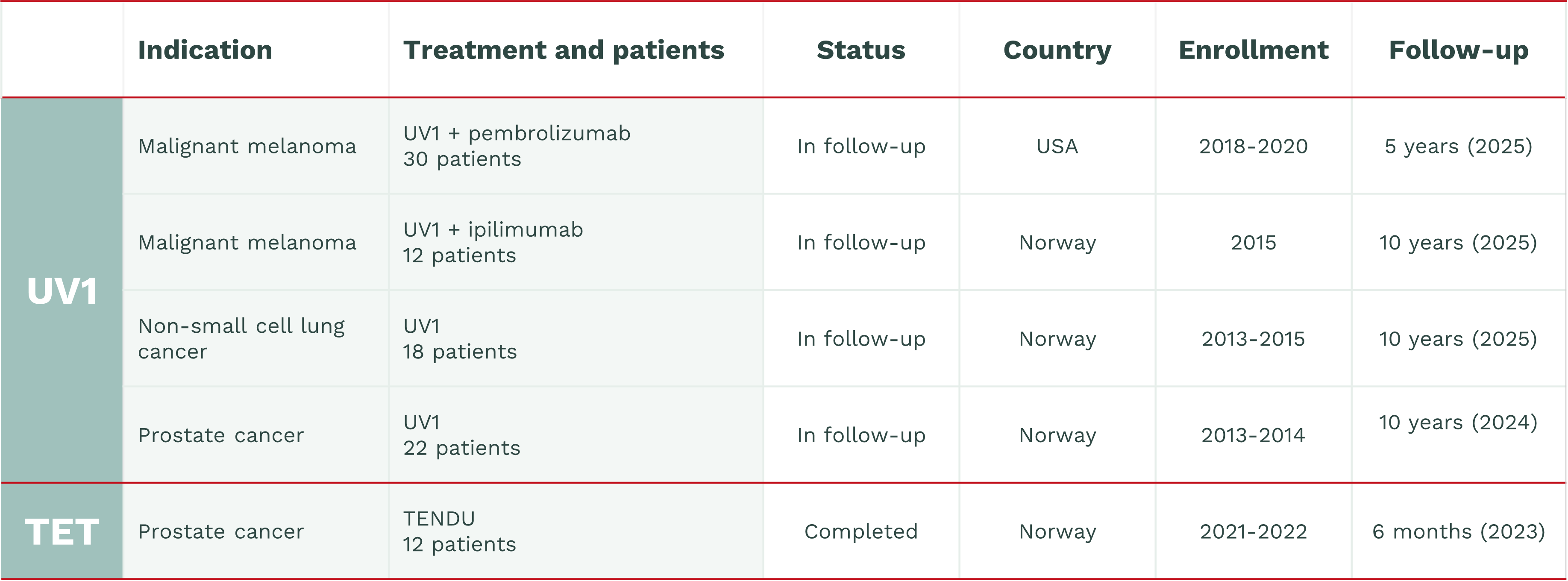
Ultimovacs is the sponsor of the fully enrolled and ongoing Phase I clinical study in the U.S. evaluating the safety and tolerability of treatment with UV1 and pembrolizumab (PD-1 checkpoint inhibitor) in 30 patients with metastatic malignant melanoma. In addition, treatment with UV1 has been assessed in three completed Phase I studies (metastatic prostate cancer, metastatic non-small cell lung cancer and metastatic malignant melanoma) in 52 patients at the Oslo University Hospital. The observed clinical outcomes from these trials served as a strong basis for the further clinical development of UV1, both with respect to safety, immune response and signals of clinical effect.
The TET technology has been investigated in one completed Phase I trial.
UV1 ongoing Phase I studies
Malignant melanoma
Ultimovacs is the sponsor of a fully enrolled and ongoing Phase I clinical study in the U.S. evaluating the safety and tolerability of treatment with UV1 and pembrolizumab (PD-1 checkpoint inhibitor) in 30 patients with metastatic malignant melanoma. Enrolment of patients started in July 2018 and was completed in August 2020.
Malignant melanoma is a type of cancer that develops from the pigment-containing cells known as melanocytes. Melanomas typically occur in the skin but may also occur in the intestines, mouth or the eyes. Worldwide over 280,000 new cases of malignant melanoma were diagnosed in 2018, and it is estimated that more than 60,000 persons died from the disease.
UV1 Phase I studies in long-term follow-up
Prostate cancer
(EudraCT No. 2012-002411-26)
Prostate patients with advanced disease without lung and/or liver metastases were enrolled. These patients had started CAB treatment (GnRH-agonist combined with anti-androgen) prior to UV1 treatment.)
Non-small cell lung cancer (NSCLC)
In the lung study stage 3b/4 NSCLC patients were enrolled, who previous had been treated with palliative radiotherapy and /or at least two courses of chemotherapy. These patients were not to be in progression, confirmed by CT, at least 4 weeks prior to UV1 treatment.
Malignant melanoma – UV1 in combination with ipilimumab
The malignant melanoma patients had unresectable or metastatic disease when enrolled, and were eligible for ipilimumab. Safety and tolerability were primary endpoints in all three studies, while immune response towards any of the UV1 peptides and efficacy were secondary endpoints. Three different dose levels of UV1 were investigated in the prostate cancer and NSCLC studies (100, 300 and 700 µg). In the malignant melanoma study, 300 µg UV1 was given in combination with ipilimumab.
Data from the three studies showed that UV1 is generally well tolerated. There were no dose limiting toxicities. UV1 induced an immune response (hTERT specific T-cells) in 78% (range 67-91%) of patients across the three studies.
When combining UV1 with ipilimumab, a CTLA-4 checkpoint inhibitor, 91% of malignant melanoma patients developed an immune response. The responses appeared earlier, required fewer vaccinations, and were stronger and more long lasting compared to vaccination with UV1 alone. These data are compatible with a mechanism of action where blocking CTLA-4 checkpoints induce additional expansion of UV1 specific T cells induced by UV1 vaccination.
TET technology Phase I studies
TENDU – prostate cancer
The TENDU trial was the first Phase I trial exploring the TET technology. In TENDU, the TET technology
incorporates prostate-cancer-specific antigens and the trial will provide valuable safety and immune
activation data that will support the further development of new vaccine solutions based on the TET
technology.
The TENDU was conducted at Oslo University Hospital with enrollment from February 2021 to December 2022. A total of 12 patients were treated at one of three doses: 40 mcg (3 patients), 400 mcg (3 patients) and 960mcg (6 patients), with follow-up for six months after last treatment.
Ultimovacs announced results from the TENDU study in December 2023. The trial showed good safety and tolerability across all dose cohorts, meeting the primary endpoint. The data also included observations of immune activation with vaccine-specific T cell responses, meeting the secondary endpoint. No dose-limiting toxicities were observed, indicating a potential for increasing the dose of tetanus-based vaccines in future clinical studies. Further results from the study will be presented in a peer-reviewed publication.