NOTICE OF ANNUAL GENERAL MEETING IN ZELLUNA ASA
The Board of Directors hereby calls for the Annual General Meeting in Zelluna ASA at 14:00 CET on 29 April 2025. Please go to the Governance page for all documents related to the Annual General Meeting.
The General Meeting will be held electronically. For participation, please log in at https://dnb.lumiagm.com/113357304 (Log in BEFORE the meeting starts at 14:00 CET) You must identify yourself through user ID and password which has been sent to you.
For shareholders receiving notice by post (with Ref.nr and PIN) enrollment, advance votes and Proxy may be registered here (deadline 25 April 2025 16:00 CET )
Shareholders receiving notice electronically, must log on Investor services and choose Corporate Actions – General Meeting – ISIN (also available for shareholders receiving notice by post)
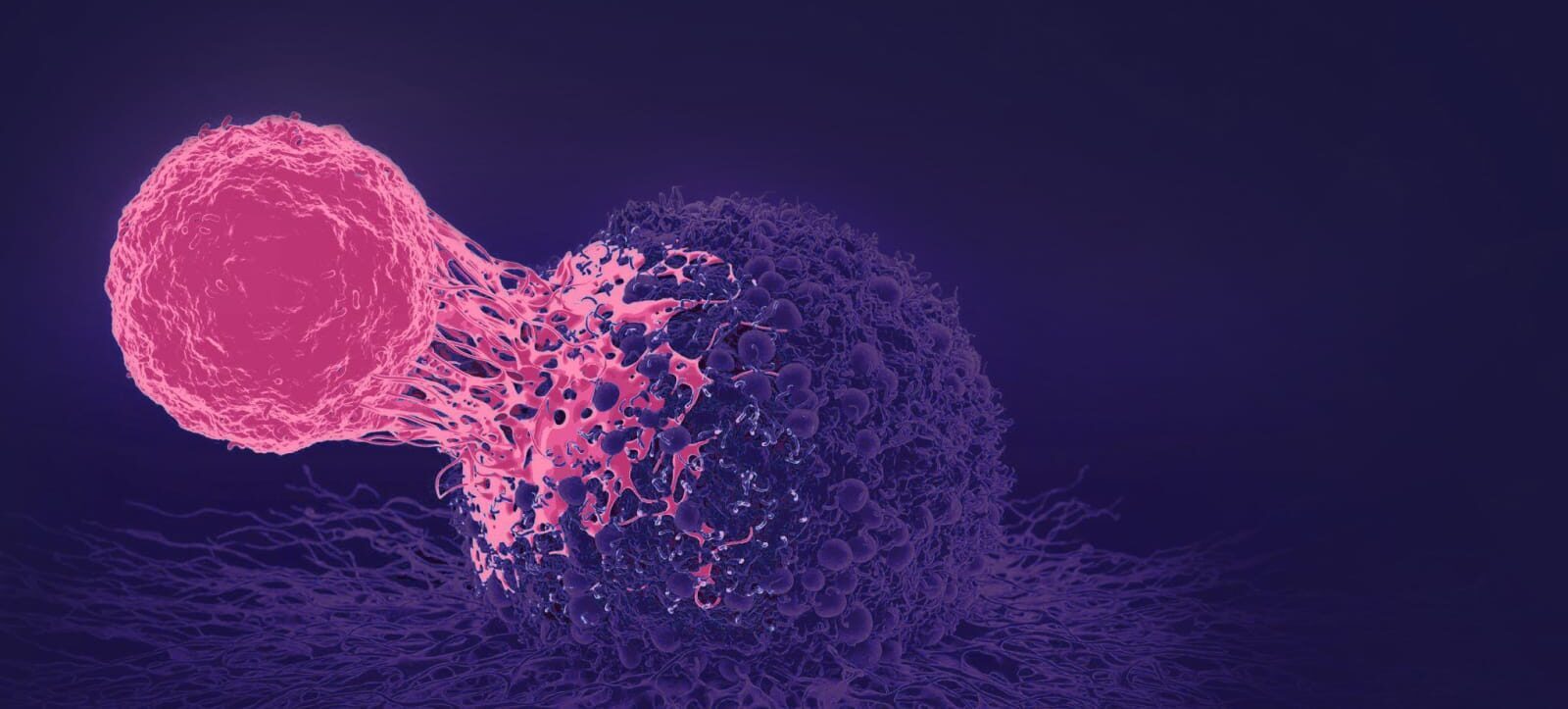
Pioneering novel cell therapies for the treatment of cancer patients
Cell Therapies Can “Cure” Cancers
Cell therapies have delivered “cures” in certain late-stage cancer patients with multiple approved treatments mainly for liquid cancers. Several cell therapy products have been approved by regulatory authorities with data from less than 100 patients.
Solid Cancers remain a major challenge
Whilst there has been progress, successes in liquid cancers have not translated into solid cancers which represents by far the largest cancer burden and remains an unsolved challenge
Zelluna targets Solid Cancers
Zelluna is developing a unique and proprietary TCR-NK cell therapy platform.
TCR-NK therapies are designed to overcome the current challenges of treating solid cancers and can potentially be used to treat a range of solid cancers with unmet medical need in high value markets.
Saving life through innovative cancer targeted therapies
Zelluna’s mission is to eliminate solid cancers by unleashing the most powerful elements of the immune system through pioneering the development of T cell receptor (TCR) guided natural killer (NK) cell therapies. The team comprises experienced biotech entrepreneurs that have taken immune-oncology projects from inception through to the clinic and supported by a highly experienced international board