The UV1 vaccine
Technology
The UV1 vaccine consists of three long synthetic peptides, representing 60 amino acids of the reverse transcriptase subunit of human telomerase (hTERT). The UV1 peptides contain several CD4 and CD8 epitopes, shown to be promiscuous in terms of Human Leukocyte Antigen (HLA) allele type for presentation. It is therefore not required to perform HLA pre-screening of patients to achieve a broad population coverage of the vaccine. UV1 is administered by repeated intradermal injections together with the immune-modulator GM-CSF.
Telomerase (hTERT)
Telomerase is essential for the ability of cancer cells to proliferate beyond the limits of normal cells. The enzyme is active in over 85-90% of all cancer cell types, but inactive in most normal cells. Since telomerase is essential for cancer cell immortality and universal expressed by most types of cancer cells, it is an ideal antigen in immunotherapy for cancer.
UV1 mode of action
The mechanism of action for the UV1 vaccine is to induce a specific T cell response against hTERT. Following intradermal injection, professional antigen presenting cells (APCs) in the skin are exposed to the synthetic vaccine peptides corresponding to hTERT degradation products associated with cancer. These APCs will process the vaccine peptides, and present antigenic determinants in a HLA-restricted fashion to näive T cells in the lymph nodes. Activated vaccine specific T cells will then enter the circulation and search for cells displaying their cognate antigen in the context of HLA complexes.
UV1 consist of long, synthetic peptides shown to induce CD4+ T cells displaying a Th1 cytokine profile. These cells have the potential to provide the inflammatory signals and T cell help thought to be critical for triggering of a strong anti-tumor immune response.
The UV1 vaccine peptides
As opposed to algorithm-selected vaccine peptides, the UV1 peptides contain epitopes documented to be recognized by the immune system of cancer patients. The strategy for selection of these peptides include screening of a hTERT peptide library using blood samples collected from cancer patients who responded immunologically to treatment with different first-generation hTERT vaccines. Immune responses against several hTERT epitopes including novel hTERT epitopes not present in the vaccines given, were detected in blood samples from long-term surviving patients following vaccine treatment, but not in patients without clinical benefit. Based on these data, three hTERT peptides shown to elicit strong T cell responses across different cancer types were selected as components for the UV1 vaccine. UV1 is thus the first cancer vaccine based on epitope spreading data, and patient data associate UV1 peptide responses with survival benefit.
The TET technology
TET (Tetanus-Epitope Targeting) is Ultimovacs’ patent protected vaccine adjuvant technology. TET ensures targeted delivery of both antigen and adjuvant signals to antigen presenting cells, and is a novel strategy to effectively activate tumor specific T cells.
TET vaccines have the tumor antigen and the adjuvanting signals in one unit.
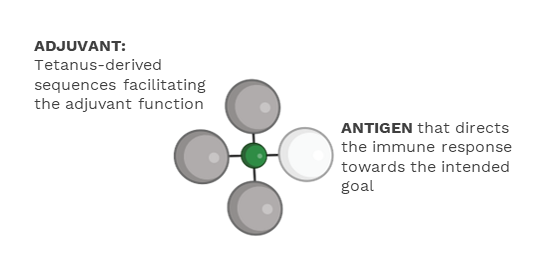
The adjuvanting effect is mediated by the tetanus- derived sequences. TET harness the immune activation function of immune complexes that is formed between the tetanus-derived parts of the vaccine and pre-existing antibodies against tetanus resulting from standard tetanus vaccination. Immune complex formation is known to be an effective way to initiate and amplify an immune response.
In Ultimovacs’ TET vaccines, the tetanus sequences and the antigen are linked by use of an innovative conjugation technology. This conjugation technology allows for flexibility to incorporate a variety of antigens, and thereby tailoring vaccines to different types of cancer. The TET vaccine adjuvant technology and the conjugation technology may be basis for new, first-in-class therapeutic cancer vaccines.
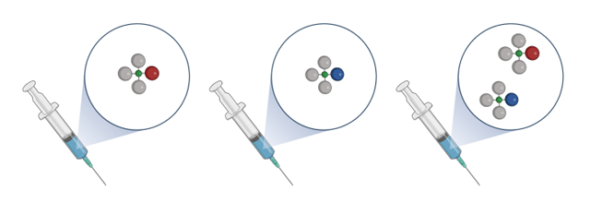
Ultimovacs is conducting a series of activities to further develop and explore the potential of TET and the conjugation technology. Pre-clinical experiments support the TET strategy of targeted delivery of antigens and adjuvant signals to antigen presenting cells. The combination of exploratory research using Ultimovacs’ conjugation technology, significant progress made in the manufacturing process, and the clinical data, provide a valuable basis for potential expansion of Ultimovacs’ pipeline. Ultimovacs will continue the ongoing TET nonclinical activities. Future development of TET based vaccine candidates will take into consideration the evolution of the therapeutic landscape and medical needs in different tumor types.